
by Steph Dunning | May 23, 2025 | News
Learn More about...
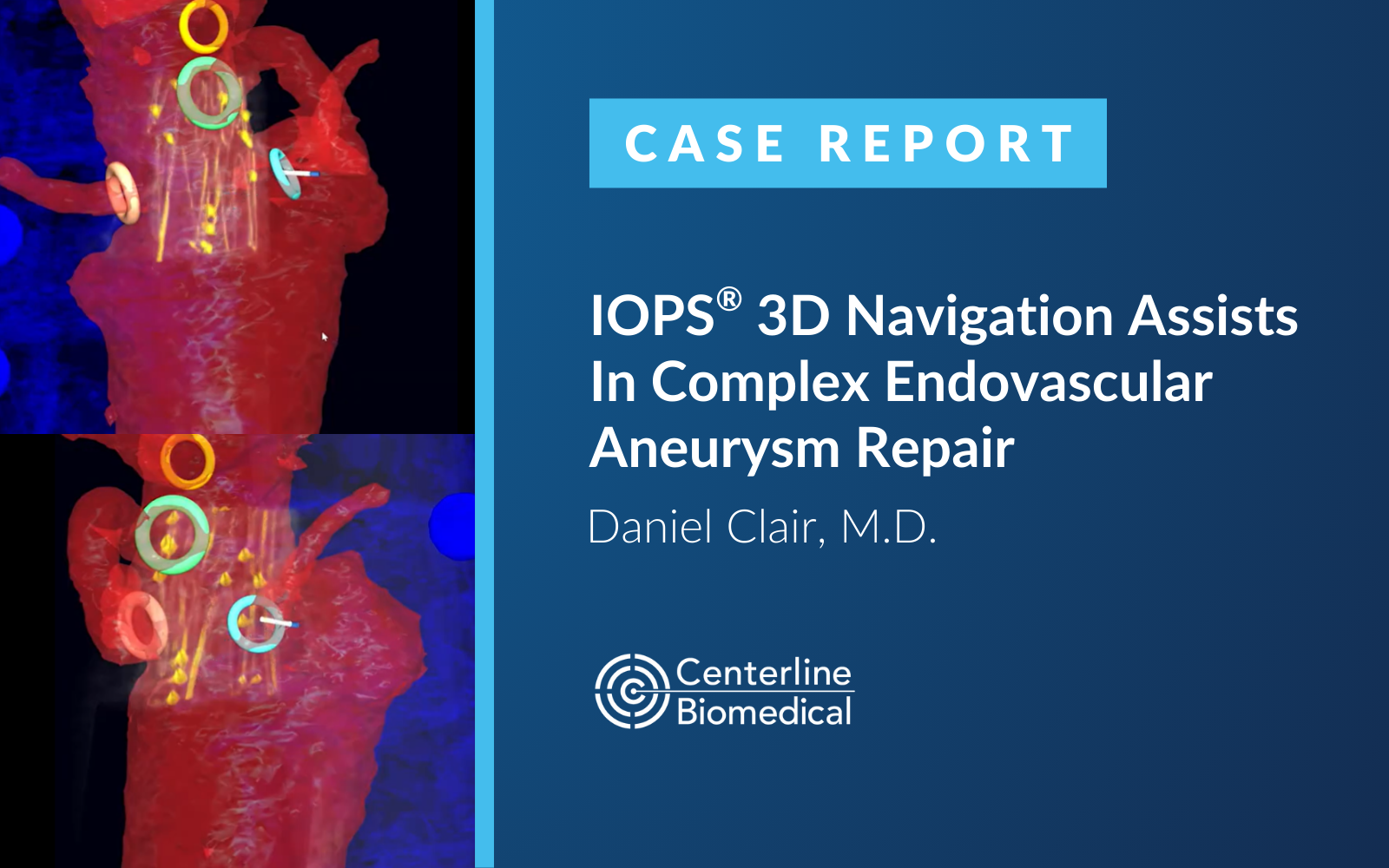
by Steph Dunning | May 16, 2025 | Case Study
LEAD CLINICIANDaniel Clair, M.D.Professor and Chair, Dept. of Vascular SurgeryVanderbilt University Medical Center (Nashville, Tennessee) PROCEDURE TYPE:Complex Endovascular Aneurysm Repair (EVAR) with Zenith Fenestrated Endovascular Graft (ZFen) BRIEF:A complex EVAR...
by Steph Dunning | Mar 11, 2025 | News
CLEVELAND, March 11, 2025 – Centerline Biomedical, Inc. (“Centerline”), an innovative leader in cardiovascular navigation and visualization systems, announced that the company has received US Food and Drug Administration (FDA) 510(k) clearance of expanded indications...

by Steph Dunning | Dec 4, 2024 | News
CLEVELAND, Dec. 4, 2024 /PRNewswire/ — Centerline Biomedical, Inc. (“Centerline”), an innovative leader in cardiovascular navigation and visualization systems, announced that the company has received US Food and Drug Administration (FDA) 510(k)...
by Steph Dunning | Nov 19, 2024 | News
CLEVELAND, Nov. 19, 2024 /PRNewswire/ — Centerline Biomedical, Inc. (“Centerline”), an innovation leader in cardiovascular navigation and visualization systems, announced today the first on-label use of the 6Fr Viewpoint Catheter as part of the...







Recent Comments